|
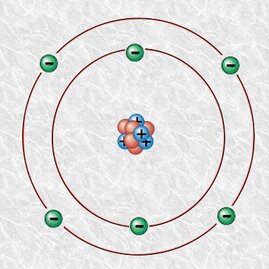
Stars begin as enormous fusion reactors. At first, hydrogen atoms fuse to become helium atoms—with a tiny bit of matter being converted to energy in the process. That tiny bit produces lots and lots of energy; it’s like a huge, continuous hydrogen bomb explosion. Toward the end of a star’s life, it’s rich in complex elements formed by additional fusion reactions. One of these elements is carbon. Carbon is the champion of living things. Without carbon, there would be no life on Earth.
In this way, carbon and other heavy elements landed in our solar system. We truly are stardust.
Diamonds are pure carbon in a transparent, crystalline structure that gives it both hardness and resistance to heat. A diamond melts at 4,000°C (7,232°F), and is the hardest naturally occurring substance on Earth. Graphite is also pure carbon, but in a different crystal lattice arranged in layers. Graphite has about the same melting point as diamond, but it’s soft and slippery because its layers can slide under physical pressure; for this reason, graphite is useful in lubrication and as pencil “lead.” Within your body, carbon is vitally important to proteins that form your skin, bones, muscles, brain, and other organs, and it is found in small molecules, hormones, and large-stranded DNA. Carbohydrates are energy-rich molecules which, as the name tells you, consist of carbon combined with water (H2O). Fats consist of long chains of carbon atoms with hydrogen atoms attached; the bonds between the carbons store energy. Every tissue, structure, and process in Earth’s living organisms depends on carbon. The chemistry of carbon is called organic chemistry for this reason. Carbon doesn’t last forever, but for our purposes, it’s close. All the carbon that arrived as the Earth was forming so long ago is pretty much still here. It cycles through both the living and inanimate worlds; plants ultimately build their bodies from the carbon in carbon dioxide, and animals from eating other organisms. We are all exchanging carbon all the time. Some of the carbon atoms in your body may have cycled from an ancient ocean or forest, or from long-dead creatures as yet unknown. Some of the carbon you take in may have resided most recently in your ancestors, friends, or even your pets—whether carried in the air you breathe or having been incorporated into the food you ate for lunch. Whatever the case, may carbon be with you. It unites us all. This article is dedicated to my long-time friend Deborah Robbins.
6 Comments
|
Categories
All
Archives
January 2024
|



 RSS Feed
RSS Feed