|
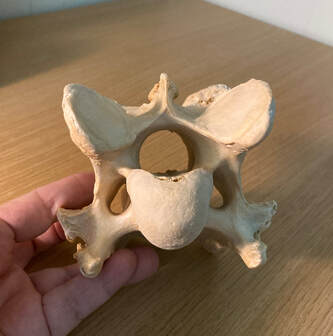
Vertebrates are animals with hard internal skeletons—and specifically, backbones. The backbone or spine is a series of bony vertebrae that protect the spinal cord and the nerves that branch from it. Vertebrates include bony fishes, all amphibians and reptiles, birds, and mammals. Sharks and their relatives are vertebrates too, but their skeletons are composed of cartilage instead of bone.
This first view is from the end that points toward the head. The back of the neck would be on top, and the front of the neck on the bottom. The opening in the middle is where the spinal cord passes through, and the smaller openings on each side are for spinal nerves. The rounded, triangular knob is part of the top intervertebral joint of the vertebral body, the part which supports the most weight. The two smooth flat areas on each side above the knob make up part of the facet joints that also connect the vertebrae.
Something unexpected happened when I set the vertebra down on its tail end. I found that it had a surprise—one that was hiding in plain sight until this moment. The bony structure was the same as it had always been, but here was something new. Do you see it? “Numinous” is an older word, and uncommon now. It refers to something that is spiritually elevated, supernatural, incapable of being described or understood; mysterious. It’s from “numen,” a Latin word, meaning a presiding divinity or the spirit of a place. Numen may also mean creative energy or genius.
Do I believe there is literally an “angel” in this vertebra? No—but I do think my seeing an angel in this shape is no accident. Human brains and minds have also been molded by function—and culture—for tens of thousands if not millions of years. This “angel” is an example of a different level of meaning residing in common objects. It’s numinous. P.S. If you saw something other than an angel, no problem. Whatever you saw is right.
4 Comments
My previous post was in praise of carbon, that versatile element—wonderful, flexible carbon, which came from an exploding star. Ninety-four elements came from that star, and among them was oxygen. Oxygen is highly reactive—it quickly combines with other elements to form compounds called oxides, such as carbon dioxide (CO2). You can see this reaction taking place anytime you have a fire. During the first couple of billion years of the Earth’s existence, oxygen was pretty much tied up in oxides all the time. Any free oxygen, or O2, would quickly combine with other elements. Earth’s atmosphere is thought to have been mostly carbon dioxide, methane, and water. Around 3 billion years ago, a variety of life forms began to appear in the shallow oceans that covered Earth. We know very little about them, because they barely even made fossils. We know only what scientists can infer from microfossils, from presumed modern descendants, and from the mineral composition of deposits laid down way back then, when they can be found.
It’s thought that free oxygen wiped out a vast number of life forms globally. This was a slow-motion extinction compared to the asteroid impact that killed the dinosaurs, but it was still disastrous for life at that time. Because there is so little direct evidence, scientists don’t technically consider it a “mass extinction.” The life forms that survived were able to detoxify oxygen, and even used it to power biochemical processes. As time went on, some of these cells engulfed the early cyanobacteria. Instead of being broken down for energy, some cyanobacteria continued to live inside an engulfing cell—a process termed endosymbiosis that eventually led to the rise of true plants. Chloroplasts are descendants of those ancient cyanobacteria. Plants not only produce oxygen; they also consume carbon dioxide. On land, large forests are particularly good at this. Reforestation and preservation of existing forests could help offset carbon dioxide produced from burning fossil fuels.
With proper strategies, the rise in atmospheric CO2 and the resulting greenhouse effect and climate change could be slowed or stopped. If we do nothing, who knows? Depending on how severe climate change gets, recovery could take a few hundred million years, more or less—but the Earth has plenty of time. For Further Exploration
[The articles listed below are somewhat technical. Wikipedia actually contains some fairly solid, basic information on the Great Oxidation Event.] Robert E. Blankenship, “Early Evolution of Photosynthesis.” Plant Physiology, 2010 Oct; 154(2): 434–438. Click here. Roger Buick, “When did oxygenic photosynthesis evolve?” Philosophical Transactions of the Royal Society B: Biological Sciences, 2008 Aug 27; 363(1504): 2731–2743. Click here. Michael W. Gray and Keith G. Kozminski, “Lynn Margulis and the endosymbiont hypothesis: 50 years later.” Mol Biol Cell. 2017 May 15; 28(10): 1285–1287. [This gives a summary of endosymbiosis as described by Lynn Margulis.] Click here. Katharine Rooney, “These tiny plants and giant animals are helping to store vast amounts of CO2 in our oceans.” World Economic Forum, May 19, 2021. [This is a mostly non-technical article that talks about diatoms, but also about whales.] Click here. Stars begin as enormous fusion reactors. At first, hydrogen atoms fuse to become helium atoms—with a tiny bit of matter being converted to energy in the process. That tiny bit produces lots and lots of energy; it’s like a huge, continuous hydrogen bomb explosion. Toward the end of a star’s life, it’s rich in complex elements formed by additional fusion reactions. One of these elements is carbon. Carbon is the champion of living things. Without carbon, there would be no life on Earth.
In this way, carbon and other heavy elements landed in our solar system. We truly are stardust.
Diamonds are pure carbon in a transparent, crystalline structure that gives it both hardness and resistance to heat. A diamond melts at 4,000°C (7,232°F), and is the hardest naturally occurring substance on Earth. Graphite is also pure carbon, but in a different crystal lattice arranged in layers. Graphite has about the same melting point as diamond, but it’s soft and slippery because its layers can slide under physical pressure; for this reason, graphite is useful in lubrication and as pencil “lead.” Within your body, carbon is vitally important to proteins that form your skin, bones, muscles, brain, and other organs, and it is found in small molecules, hormones, and large-stranded DNA. Carbohydrates are energy-rich molecules which, as the name tells you, consist of carbon combined with water (H2O). Fats consist of long chains of carbon atoms with hydrogen atoms attached; the bonds between the carbons store energy. Every tissue, structure, and process in Earth’s living organisms depends on carbon. The chemistry of carbon is called organic chemistry for this reason. Carbon doesn’t last forever, but for our purposes, it’s close. All the carbon that arrived as the Earth was forming so long ago is pretty much still here. It cycles through both the living and inanimate worlds; plants ultimately build their bodies from the carbon in carbon dioxide, and animals from eating other organisms. We are all exchanging carbon all the time. Some of the carbon atoms in your body may have cycled from an ancient ocean or forest, or from long-dead creatures as yet unknown. Some of the carbon you take in may have resided most recently in your ancestors, friends, or even your pets—whether carried in the air you breathe or having been incorporated into the food you ate for lunch. Whatever the case, may carbon be with you. It unites us all. This article is dedicated to my long-time friend Deborah Robbins.
Over those thousands of years, any genetic differences that gave an individual an advantage in the high-altitude environment tended to be preserved and to increase in the population. This is the classic concept of natural selection. Tibetans are not the only people who live up high. The Aymara people of the Andes live at an average elevation of about 13,000 ft (4,000 meters). Humans moved into the Andes perhaps 11,000 years ago. And, the Amhara people of northwestern Ethiopia live at an average elevation of 10,663 ft (3,250 meters). Anthropologist Cynthia Beall of Case Western Reserve University has spent decades studying the physiology of high-altitude populations. Here are a few of the physiological differences among Tibetans, Andeans, and Ethiopians, based on her work and that of others: Tibetans:
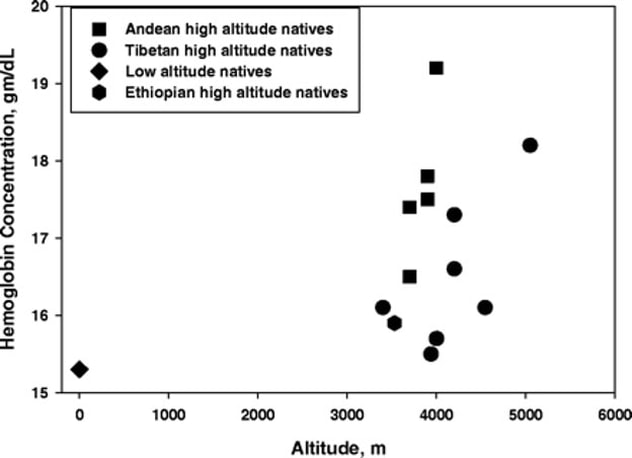
It’s puzzling how Tibetans manage to keep their physiological oxygen high enough since they don’t show increased hemoglobin or increased oxygen in hemoglobin. Basically, Tibetans tolerate having a low oxygen level in their arterial blood, and yet their tissues manage to utilize the oxygen they need. Ethiopians are another puzzle; their hemoglobin and oxygen-in-hemoglobin measurements are close to that of lowlanders, but they too are able to thrive in the highlands. The graph below illustrates hemoglobin versus altitude. Notice that low-altitude natives are shown down in the lower left corner, and that the single point for Ethiopians is between 3000 and 4000 m altitude in the lower part of the figure. Naturally, scientists are looking for clues. In the last ten years, a gene known as EPAS1 has been studied extensively. This gene plays a role in the production of erythropoietin (EPO), which controls production of red blood cells. When oxygen is low, EPAS1 transcription is turned on and stimulates EPO production. This in turn leads to increased red blood cells and thus greater oxygen availability. You may remember the Tour de France scandals of the early 2000s that involved the use of EPO by competitors as a performance enhancing drug. When lowlanders travel to high altitude, EPO increases, and this is thought to compensate for lowered oxygen availability. In Andeans living at altitude, increased EPO is a way of life. But Tibetans and Ethiopians do not show increased EPO. Because of their elevated red blood cell count, Andean natives are at risk for developing chronic mountain sickness. This ailment is a result of increased blood viscosity, which slows circulation—particularly through the lungs. These people may suffer from low oxygen in spite of high hemoglobin. Tibetans and Ethiopians, in contrast, are more likely to avoid this condition. In Tibetans, the EPAS1 gene is altered. Although it does stimulate production of EPO, it does so at a lower level. What’s more, this particular altered EPAS1 gene is found almost exclusively in Tibetans. (A small percentage of Han Chinese also carry this altered gene.) So far, Ethiopians do not show an altered EPAS1, but they do show altered versions of some other genes in the hemoglobin and red-cell pathways. Here’s another twist to the story: the altered EPAS1 of Tibetans has evidently come from a group of hominins (closely related, archaic humans) known as the Denisovans, who went extinct about 40,000 years ago. Not much is known about Denisovans because only a few fragmentary fossils have been found. DNA analysis, however, shows that they were distinct from Neanderthals and from modern humans. Interbreeding occurred among these groups on multiple occasions over thousands of years. The altered EPAS1 has no particular effect at low altitude, which may be why it has been preserved in Tibetans and Sherpa people. Adaptation to high-altitude environments has apparently taken more than one path in humans. Scientist are continuing to investigate other possibilities to explain how Tibetans, and Sherpas in particular, are able to live the high life. For further exploration
[These first four are technical articles:] Cynthia M. Beall, “Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia.” Integrative and Comparative Biology, 46(1), February 2006. Click here. Cynthia M. Beall, “Two routes to functional adaptation: Tibetan and Andean high-altitude natives.” Proceedings of the National Academy of Sciences (PNAS), May 15, 2007. Click here. Cynthia M. Beall, Michael J. Decker, Gary M. Brittenham, et al. “An Ethiopian pattern of human adaptation to high-altitude hypoxia.” Proceedings of the National Academy of Sciences (PNAS), December 2002. Click here. Masayuki Hanaoka, Yunden Droma, Buddha Basnyat, et al. “Genetic Variants in EPAS1 Contribute to Adaptation to High-Altitude Hypoxia in Sherpas.” PLoS ONE 7(12), December 2012. Click here. [Nontechnical:] Ann Gibbons, “Tibetans Inherited High-Altitude Gene from Ancient Human.” Science, July 2, 2014. Click here. Mark Synnott, The Third Pole. New York: Dutton, Penguin Random House LLC, 2021. [This book describes the author’s journey to Everest in search of the remains of Sandy Irvine. Along the way he tells us a lot about the George Mallory expedition, about other Everest climbers, and about the Sherpas’ abilities. It was this book that introduced me to the Denisovan EPAS1 gene.] “Burbank was a plant mystic who created new plants with little more than his imagination, his pollinating abilities, and his skill for selection.” —Jules Janick Luther Burbank was born in 1849 in Lancaster, Massachusetts, to Samuel Burbank and his third wife, Olive Burpee Ross. The thirteenth of his father’s fifteen children, Luther was drawn to plants and flowers from an early age. A flower could give him greater pleasure than any other kind of toy, according to biographer Henry Smith Williams.
George Harrison Shull, a biologist who worked with corn, reviewed Burbank’s work in the early 1900s and determined that Burbank’s processes were “more art than science.” Luther Burbank’s role in the creation and development of the Russet Burbank potato was his first great success. In his early 20s, he read Charles Darwin’s book The Variation of Animals and Plants Under Domestication and was impressed by the ideas. He also purchased a 17-acre farm where he planned to grow vegetables and also to “accelerate evolutionary changes,” according to biographer Jules Janick.
Cultivated potatoes depend more on their tubers than on flowers and fruit for reproduction, and the appearance of an actual seedpod is relatively rare. Inside this single seedpod were twenty-three seeds. Burbank planted all twenty-three to see what would come of them. One plant produced large, excellent tubers that tasted good and could be stored for a long time. This was the “Burbank” potato.
Burbank had a connection with plants that went beyond intellectual curiosity. He did have a method for producing his hybrids, but I think that the plants responded to his positive attention and connection as well. I’m not saying that plants have emotions or animal-like consciousness; I am no fan of anthropomorphizing. Plants do not have brains or nervous systems in the animal sense. Plants do respond to stimuli from their environment, however, and part of those stimuli may come from organisms around them, including humans. Just because we have no explanation at this moment doesn’t mean the idea is impossible. In any case, Burbank loved plants, and he certainly reaped rewards from his wizard-like work with them. For further exploration
Michael Pollan has written a fairly well-balanced article about the topic of plant “intelligence”—it appeared in the December 23 & 30, 2013, issue of The New Yorker. You can read it here. Jules Janick, “Luther Burbank: Plant Breeding Artist, Horticulturist, and Legend.” HortScience Vol. 50(2), February 2015. American Society for Horticultural Science. You can read and download a PDF here. Henry Smith Williams, Luther Burbank, His Life and Work. New York: Hearst’s International Library Co. (1915). Available in ebook/Kindle form from HardPress, Miami, FL (2017). |
Categories
All
Archives
January 2024
|

















 RSS Feed
RSS Feed