|
My previous post was in praise of carbon, that versatile element—wonderful, flexible carbon, which came from an exploding star. Ninety-four elements came from that star, and among them was oxygen. Oxygen is highly reactive—it quickly combines with other elements to form compounds called oxides, such as carbon dioxide (CO2). You can see this reaction taking place anytime you have a fire. During the first couple of billion years of the Earth’s existence, oxygen was pretty much tied up in oxides all the time. Any free oxygen, or O2, would quickly combine with other elements. Earth’s atmosphere is thought to have been mostly carbon dioxide, methane, and water. Around 3 billion years ago, a variety of life forms began to appear in the shallow oceans that covered Earth. We know very little about them, because they barely even made fossils. We know only what scientists can infer from microfossils, from presumed modern descendants, and from the mineral composition of deposits laid down way back then, when they can be found.
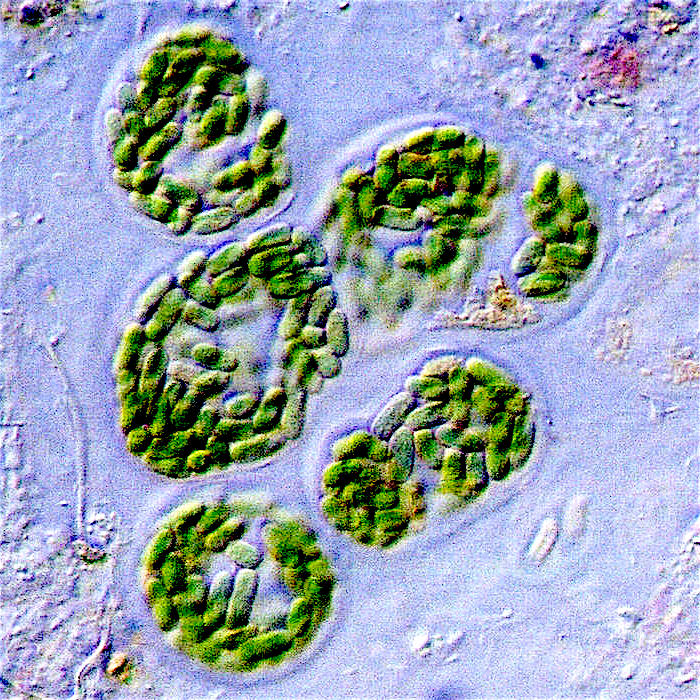
It’s thought that free oxygen wiped out a vast number of life forms globally. This was a slow-motion extinction compared to the asteroid impact that killed the dinosaurs, but it was still disastrous for life at that time. Because there is so little direct evidence, scientists don’t technically consider it a “mass extinction.” The life forms that survived were able to detoxify oxygen, and even used it to power biochemical processes. As time went on, some of these cells engulfed the early cyanobacteria. Instead of being broken down for energy, some cyanobacteria continued to live inside an engulfing cell—a process termed endosymbiosis that eventually led to the rise of true plants. Chloroplasts are descendants of those ancient cyanobacteria. Plants not only produce oxygen; they also consume carbon dioxide. On land, large forests are particularly good at this. Reforestation and preservation of existing forests could help offset carbon dioxide produced from burning fossil fuels.
With proper strategies, the rise in atmospheric CO2 and the resulting greenhouse effect and climate change could be slowed or stopped. If we do nothing, who knows? Depending on how severe climate change gets, recovery could take a few hundred million years, more or less—but the Earth has plenty of time. For Further Exploration
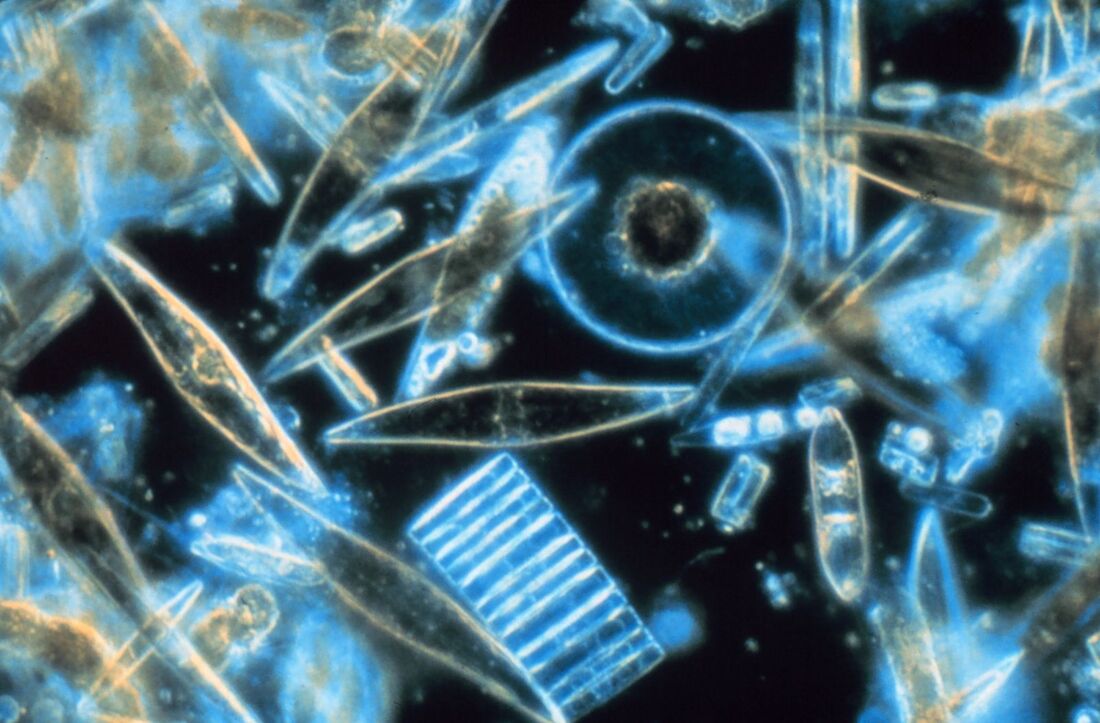
[The articles listed below are somewhat technical. Wikipedia actually contains some fairly solid, basic information on the Great Oxidation Event.] Robert E. Blankenship, “Early Evolution of Photosynthesis.” Plant Physiology, 2010 Oct; 154(2): 434–438. Click here. Roger Buick, “When did oxygenic photosynthesis evolve?” Philosophical Transactions of the Royal Society B: Biological Sciences, 2008 Aug 27; 363(1504): 2731–2743. Click here. Michael W. Gray and Keith G. Kozminski, “Lynn Margulis and the endosymbiont hypothesis: 50 years later.” Mol Biol Cell. 2017 May 15; 28(10): 1285–1287. [This gives a summary of endosymbiosis as described by Lynn Margulis.] Click here. Katharine Rooney, “These tiny plants and giant animals are helping to store vast amounts of CO2 in our oceans.” World Economic Forum, May 19, 2021. [This is a mostly non-technical article that talks about diatoms, but also about whales.] Click here.
1 Comment
|
Categories
All
Archives
January 2024
|

 RSS Feed
RSS Feed